how to calculate formal charge
Formal charge of valence electrons electrons in lone pairs 12 the number of bonding electrons Since. Formal charges are important because they allow us to predict which Lewis structure is the most likely to exist.
 |
| How To Calculate The Formal Charge Of Cocl2 Sciencing |
Of non bonding valence electrons.
. How to do formal load calculation. Now to determine the formal charge of H we will simply subtract 1 from the valence electron of H predicted by the periodic table. We can calculate the formal charge of an atom using the equation CF EV PEI ½ EL where VE the number of valence electrons of the. V is the no.
Step by Step Calculation The first step for calculating the formal charge is drawing the Lewis structure of a molecule. FC Valence electrons Nonbonding electrons- Bonding electrons2. Latextextformal chargetext valence shell electrons free atom-text lone pair electrons-frac12text bonding electronslatex. If we do we will get.
Mathematically the formal charge formula stands as follows. The formula for formal charge is. Of valence e in the free state total no. One can calculate the formal charges for any given atom with the help of the following formula.
1 F C V N B 2. Of e assigned in Lewis structure FC. A step-by-step description on how to calculate formal charges. I will teach you the super easy trick of calculating formal charge from lewis.
The formula for calculating the formal charge on an atom is simple. How to calculate formal charge using simple subtraction. The formal charge is derived from the number of valence electrons in a neutral atom. Formula to Calculate FC.
You can calculate the formal charge of any atom with the help of the equation below. The formal charge of any atom in a molecule can be calculated by the following equation. Thus we calculate formal charge as follows. Mathematically it can be expressed by the following formula.
Boron B possesses three. This lecture is about how to calculate formal charge on an atom in a molecule. Divide the electron pairs in bonds equally for all the bonds. Formal Charge number of valence electrons in neutral atom- non-bonded electrons number of bonds Take the compound BH4 or tetrahydrdoborate.
Comment below with your questions and comments - dont forget to subscribe for more videos. N is the no. 67K Dislike 530091 views Oct 25 2017 This chemistry video tutorial provides a basic introduction into how to calculate the formal charge of an atom or element in a lewis. B is the total number of electrons shared in bonds Example.
Formal Charge Valence Electrons 05Bonding Electrons Nonbonding Electrons Since the bond exists. FC Formal Charge on Atom V. FC V leftLP 05BEright Where. We can use this information to calculate the preferred Lewis structure of a molecule.
FCtextnumber of valence electrons-textnumber of lone pair electrons-textnumber of bonds Formal charge is used to determine the best.
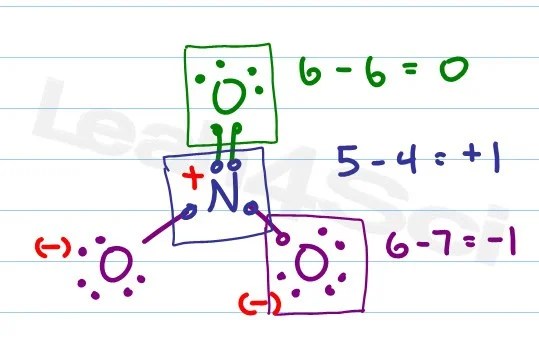 |
| No3 Resonance Structure With Formal Charge Novocom Top |
 |
| How To Show A Formal Charge For Every Atom In A Lewis Structure Quora |
 |
| Lewis Structure Formal Charge Calculation |
 |
| How To Calculate Formal Charge How To Calculate Formal Charge By Organic Chemistry Tutorial Inorganic Chemistry Science Facebook |
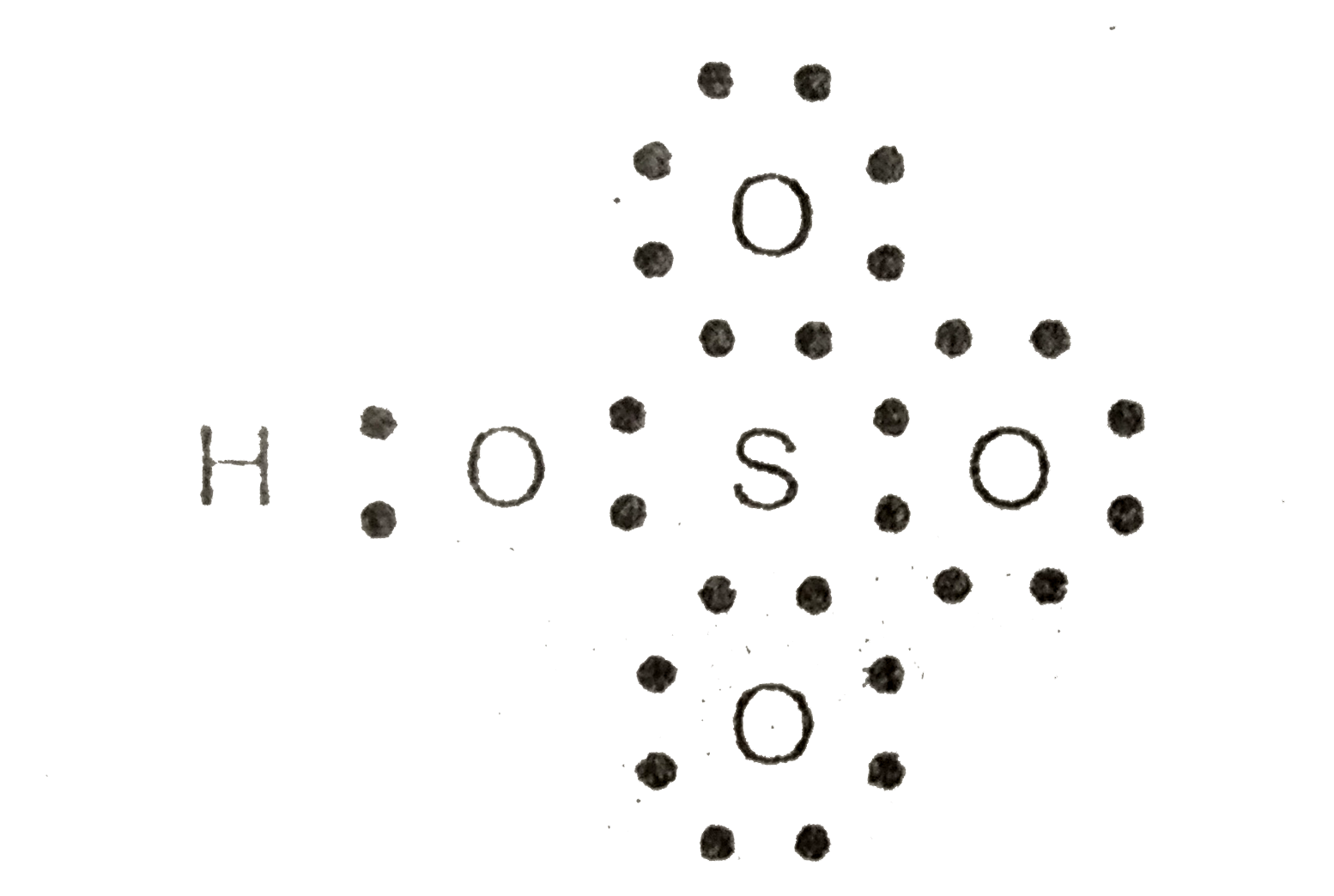 |
| Calculate The Formal Charge On S In Hso 4 Ion |
Posting Komentar untuk "how to calculate formal charge"