so3 lewis structure
Put the least electronegative atom in the center. It is an ionic compound therefore we cannot determine the lewis structure shape of the whole Na2SO3 molecule.
 |
| So3 Molecular Geometry And Bond Angles |
Mark lone pairs Step 3.
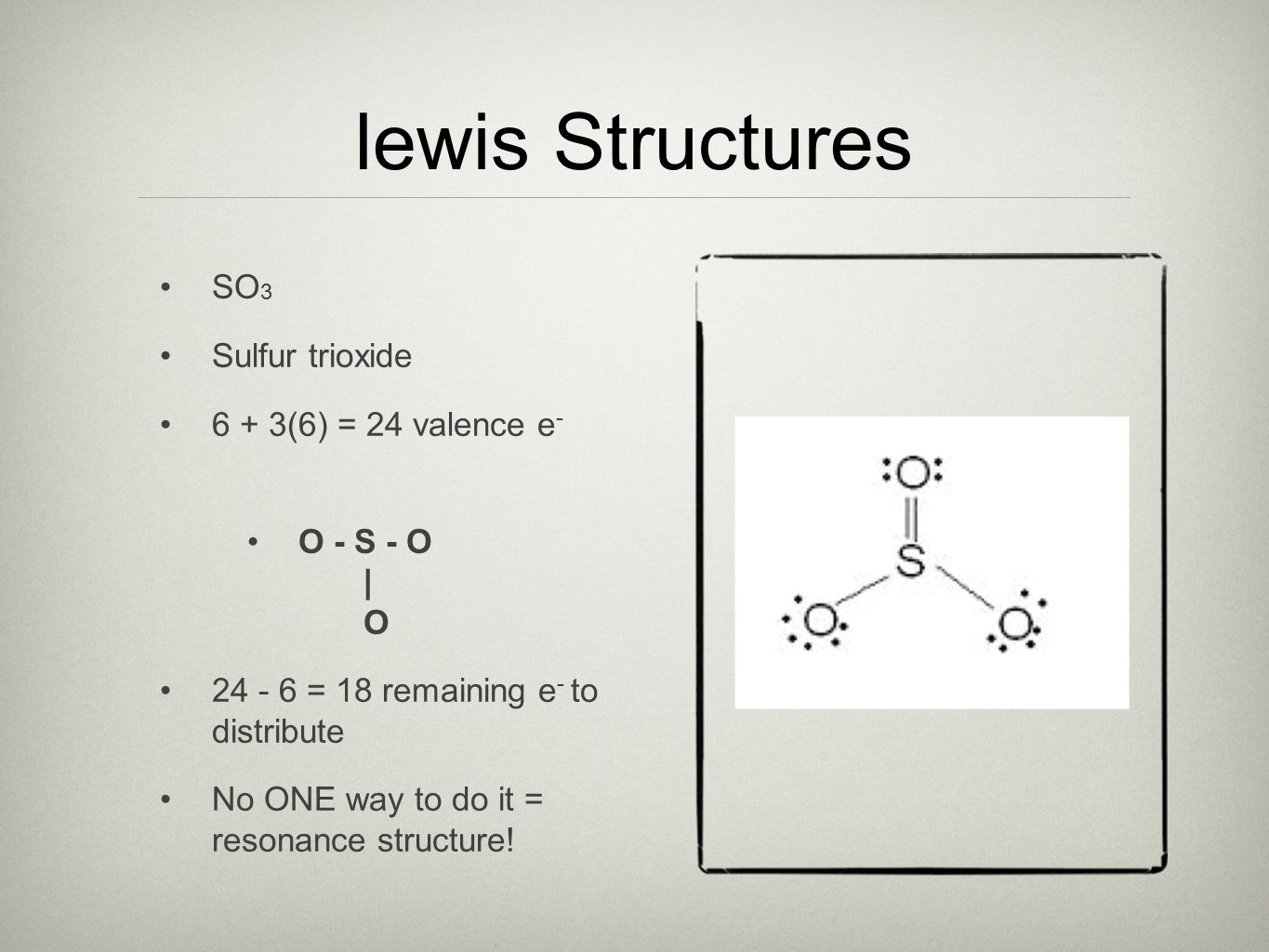
. The total number of. Find the total valence electrons in SO3 molecule. Sulfur trioxide is the chemical compound with the formula SO3. SO 3 is named Sulfur Trioxide.
In fact it can have three because Sulfur does not follow the octet rule where it tries to gain eight. It is prepared on an industrial. Does SO3 have a resonance structure. Select the central atom.
Calculate the total number of valence electrons Here the given molecule is O3 ozone. How to draw the Lewis Structure of SO3 sulfur trioxide - with explanation Sulfur is an exception to the octet rule - it can handle up to 12 electrons. In order to find the total valence electrons in SO3 sulfur. Lets do the SO3 2- Lewis structure.
Steps of drawing SO3 lewis structure Step 1. Find the total valence electrons for the SO3 2- molecule. Lewis structures can be used to represent valence shell electrons in a chemical bond. Minimize charges Step 5.
Mark charges Step 4. SO3 known as sulphur trioxide is sp2 hybridized with a triagonal planar structure and having bond angle 1200. Heres how you can draw the SO 3 lewis structure step by step. IN the periodic table.
----- Steps to Write Lewis Structure for compounds like SO3 2- ----- 1. Step 1 Figuring out the total number of valence electrons in the molecule is the first and most important step. It is a form of pollution. In order to draw the lewis structure of O3 first of all you have to find the total.
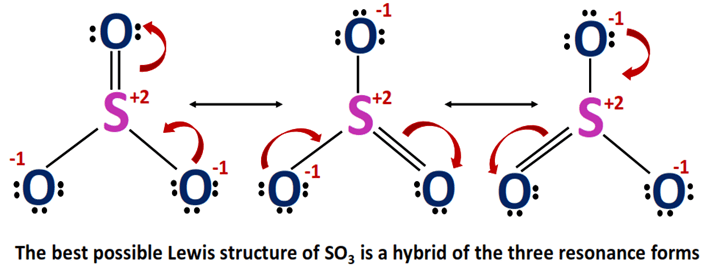
When you draw the Lewis structure you first get the three structures at the top. Here we can see that all the six electrons of the central sulfur and each oxygen are consumed in covalent bond formation. In each of them S has a formal charge. Count the total number of valence electrons present on each atom of the SO3 molecule.
Draw sketch Step 2. Trisulfur S3 CID 139340 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. For the SO3 2- compound we have 26 total valence electrons and that includes these two electrons up here--there are two extra valence electrons. SO3 has a resonance structure.
It is a colourless or white crystalline solid with boiling and melting point 450C. The Lewis structure of SO 3 is shown below. The Lewis structure proposed by Gilbert Newton Lewis who introduced it for the first time in 1916 is a. Drawing the Lewis Structure for SO 3 Sulfur Trioxide SO 3 is the primary contributer to acid rain in the atomsphere.
The Lewis structure is also called an electron dot structure which determines the number of valence electrons present in an atomMoreover they also describe how these valence. Now lets walk through the method of drawing lewis structure. There are 32 valence. SO3 Lewis Structure The sulfur trioxide SO3 is a tetra-atomic chemical molecule where three oxygen molecules and a sulfur bond have the same number of valence electrons.
There are seven resonance structures for SO_3. It has been described as unquestionably the most important economically sulfur oxide. The shape of the Na2SO3 crystal is hexagonal monoclinic. Steps for drawing Lewis dot structure of SO3 Step 1.
 |
| Solved 13 Draw Lewis Structures Of The Following Species And Name Both Course Hero |
 |
| What Are All Resonance Structures For So3 |
 |
| Write The Resonance Structures For So3 No2 And No3 |
 |
| The Top So3 2 Lewis Structure Resonance |
 |
| Calculating So3 Formal Charges Calculating Formal Charges For So3 Youtube |
Posting Komentar untuk "so3 lewis structure"